good news! And real organisms through 2021 QCMD EQA!
Recently, Heshibio obtained the QCMD 2021 Respiratory I Plus EQA Pilot Study certificate with excellent results. Respiratory I Plus EQA Pilot Study(RESPIplus) is an EQA pilot quality evaluation project following the outbreak of the new coronavirus in April 2020. The project covers the sensitivity and specificity of influenza A, influenza B, respiratory syncytial, and new coronavirus. The main goal is to evaluate tests that are routinely used for clinical SARS-CoV-2 molecular detection reagents with sensitivity close to the detection limit, and specific evaluation in the presence of other viruses.

and Real Biology passed the quality evaluation of QCMD 2021 Respiratory I Plus EQA Pilot Study with excellent results!
The reagents used in EQA are the combined respiratory tract detection kit of two rapid nucleic acid detection platforms, JeTest GeneV and JeTest Matrix.
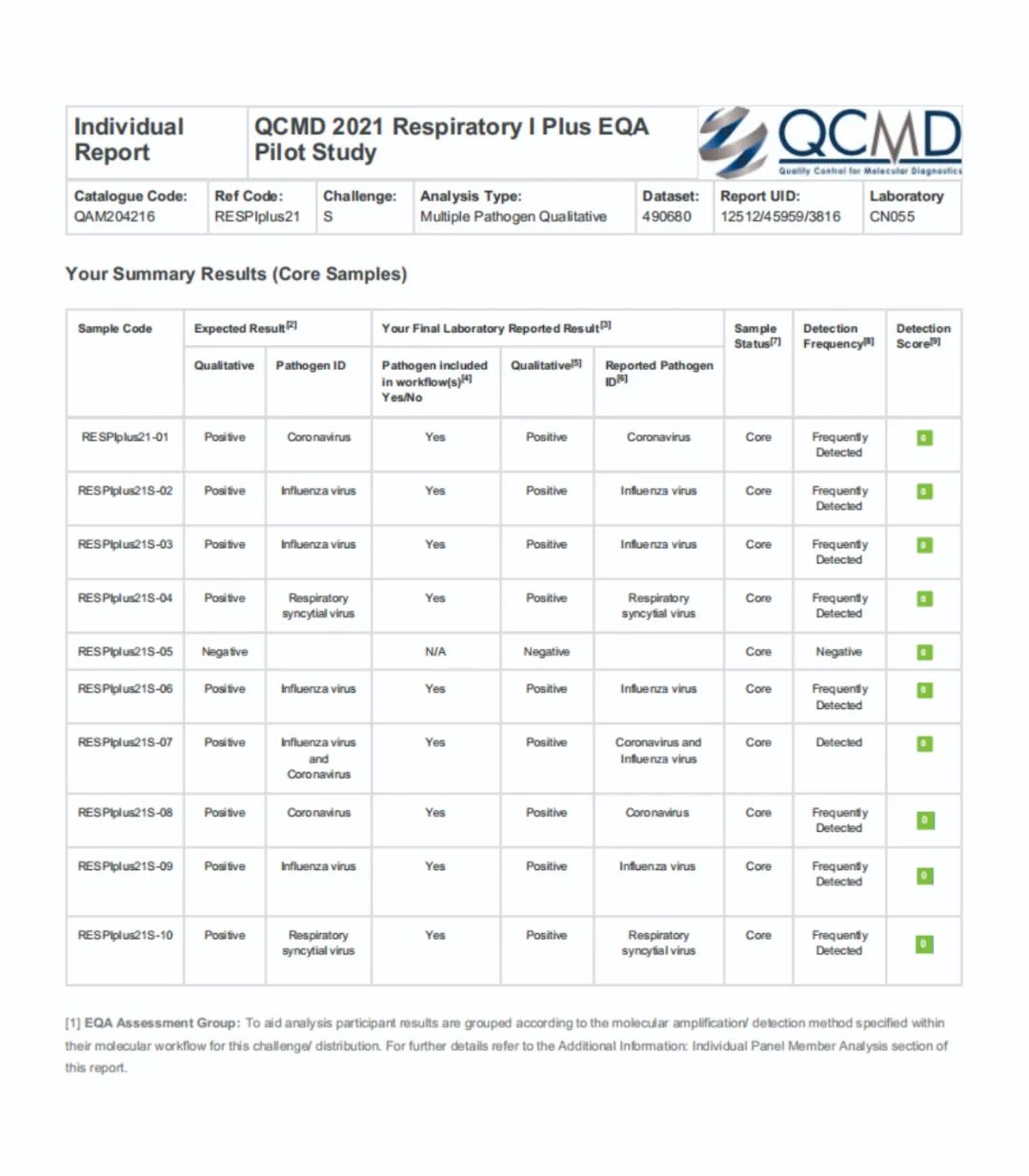
(Note: 0 is "highly satisfactory")

Quality Control for Molecular Diagnostics (QCMD), as an international EQA organization, is committed to providing professional EQA services centered on participants. Its aim is to provide participants with quality evaluation reports and practical feedback so that participants can identify and solve potential problems while monitoring the effectiveness of their laboratory quality assurance processes. QCMD was established in June 2001, and a new EQA program is introduced every year as an EQA pilot study. The successful pilot will become a complete EQA program and, where appropriate, UKAS certification will be recommended according to ISO17043:2010.
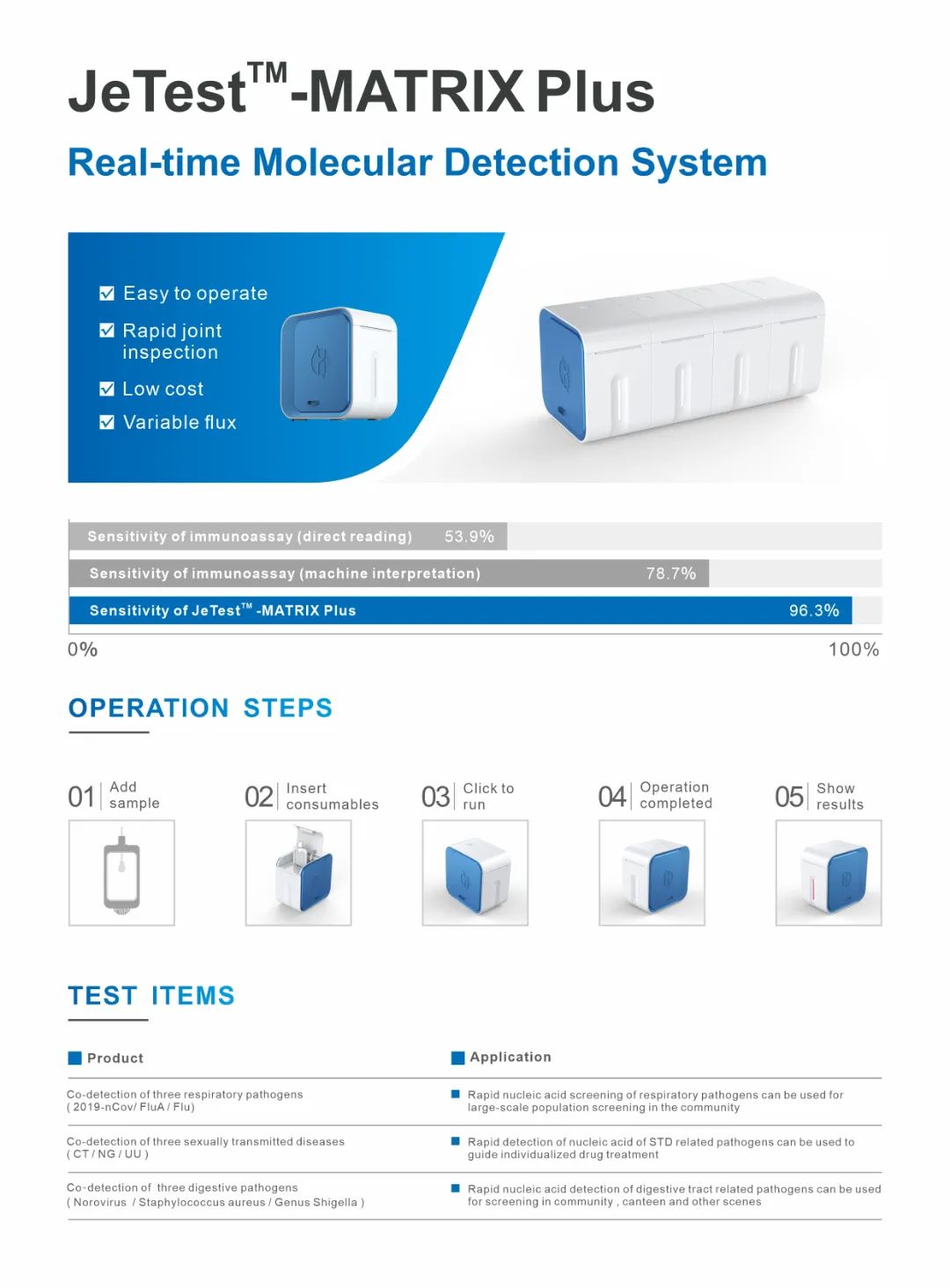
Helibio is committed to the development and application of real-time detection (molecular POCT products). At present, it has successfully launched constant temperature PCR amplification detection equipment such as Gene Jingwei V600, Gene Jingwei V800 and Matrix Plus. The appearance of the equipment is exquisite and small, the detection time is less than 30min, and it has the function of automatically interpreting the detection results. The operation is simple and convenient, and it is completely suitable for home testing and non-professionals.
In addition, Heshi Bio is also committed to the research and development of molecular POCT detection kits. Based on the constant temperature PCR amplification platform, Heshi Bio has launched new crown, new crown/A/B flow triple test, CT/NG/UU triple test and other nucleic acid detection kits that can be stored at room temperature to protect human health.

, please call us for more information.
| Product name | Specifications | number |
|
High Risk HPV DNA test (PCR Flourescent Probe Method) |
24Test/kit |
G (2013) 002 |
|
Detection kit for the Mutation of EGFR (PCR Flourescent Probe Method) |
24Test/kit |
002YG002-24 |
|
Detection kit for the Mutation of KRAS (PCR Flourescent Probe Method) |
24Test/kit |
002YG003-24 |
|
JeTest Direct 2019-nCov Detection Kit |
24Test/kit |
RT002-T24 |
|
JeTest Direct FluA/FluB/2019-nCov Detection Kit |
12Test/kit |
RT00112 |
|
JeTest Matrix plus Molecular point-of-care testing system |
Matrix Plus |
ME00601 |
|
JeTest GeneV Molecular point-of-care testing system |
V600 |
ME00724 |
|
Nucleic Acid Isolation or Purification Kit (Magnetic Beads Method) |
48Test/kit |
RT00848 |
|
Nucleic Acid Isolation or Purification Kit (Magnetic Beads Method) |
16*4 Test/kit |
RT00964 |
|
Nucleic Acid Isolation or Purification Kit (Magnetic Beads Method) |
8*8 Test/kit |
RT01064 |
|
Nucleic Acid Isolation or Purification Kit (Magnetic Beads Method) |
24Test/kit |
RT01124 |



Tel: 020-89852732
Address: Unit B, Floor 4, Building A8, No.11 Kaiyuan Avenue, Science City, Guangzhou High-tech Industrial Development Zone
mailbox: hssw@heasbio.com
website: http://www.heasbio.com



